So what is it? Well, the infinite square well is a particular choice of the Hamiltonian, or, the system. We have the usual kinetic energy term, but we have a particular potential. Imagine there's a particle. It could be an electron, a proton, a hydrogen atom. Anything small will do. We put this electron inside a very thin wire, so thin that it can only move forward or backward, not left or right or up or down. The electron can move perfectly freely inside the wire, but when it comes to one of the ends, it cannot help but bounce back. Think about you running in along a track. You keep running until you hit a wall. Since you're bored and got nothing else to do, you run the other way, until you hit the wall on the other side. You keep repeating this until you have enough bruises...
This is what the infinite square well represents. It's just an electron that's trapped along a line. We call it the infinite square well, because the wall is a barrier with infinte potential. That means you have to have infinite kinetic energy to go through! That's a pretty solid wall. It turns out, this is a decent model for electrons in a piece of metal. Here's where I think it gets cool. We solve Schrodinger's equation (SE from now on) and we get | psi >. At least, we get a way of determining how an initial state evolves with time. Let's say we put the electron in a wire and have a decent idea of where it is. It's probability density should look like a bell curve (known as the gaussian or normal distribution in probability theory.). Basically that says it's most likely at the center of the bell curve.
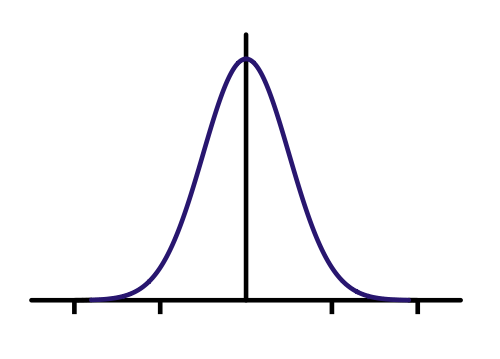
Here's a Gaussian curve. I made my initial state a bit skinnier because I think I know where I put the electron better. Let's see what SE predicts will happen to the probability density (just think of this as a guess at where the electron is, the higher the value, the more likely it could be there when we measure it.)
Pretty cool, huh? The longer you wait, the less chance you have a guessing where it it correctly. The probability density melts as time marches on. Sometimes you can see little peaks of possible places it could be. The peaks at the end are a glitch in the video that I can't seem to fix. Those frames should be at the beginning. But remember, when you solve SE, you get the wave function, not the probability density function. So what does the quantum state look like? Well hold on, this looks pretty weird I think.
There are two lines. One of the "real" part of the wave function, the other is the "imaginary" part. Don't worry too much about this, this has to do with the imaginary number "i" that I mentioned before. The wave function is very strange. I think that's cool. So now you know what happens to an electron when you set it down. But what if you give it a push? The probability density:
And the wave function (just showing the real part this time):
Let's talk about this for a second. First the probability density. It makes sense given what you just saw, right? You give the electron a kick to the right, it bounces off the wall and comes back. But what happens when you get near the wall? It gets a squiggly. Ever hear about atoms, light and other small objects acting like both waves and particles at the same time? This is where you can see it! When the electron gets near the wall, the waves that make it up interfere with each other, causing this weird squiggly effect you see when it gets near the wall! The wave function is a bit more obvious. It already looks like a wave. When parts of it bounce off the wall, they interfere with other parts. I think it looks pretty cool, what about you?
So that's it in a nutshell, there's more details that I might revisit at a later time, but they aren't that important right now. The videos were created with Python, using scipy, matplotlib, and mencoder. The source code is available if you're interested. As usual, comments and suggestions are encouraged!
Next up is the quantum mechanical version of the spring. I think this one is both really cool and important.


I love your little drawings. They really help to explain the material.
ReplyDeleteIs it possible that we look at multiple electrons in the system? I think that it would be cool to see how a system of electrons effect each other. Maybe this is more work than it is worth but I think it would be cool to see and be more applicable to the real world.
ReplyDeleteAlso, I think that you should better explain what the probability density function means as opposed to the wave function. It is just a little confusing when it comes down to what the cool animations mean. Again though, I love you animations! Nice work!
I haven't discussed multiple particle systems yet, but when I do, I'll be sure to show a multi particle wave function evolving!
ReplyDelete